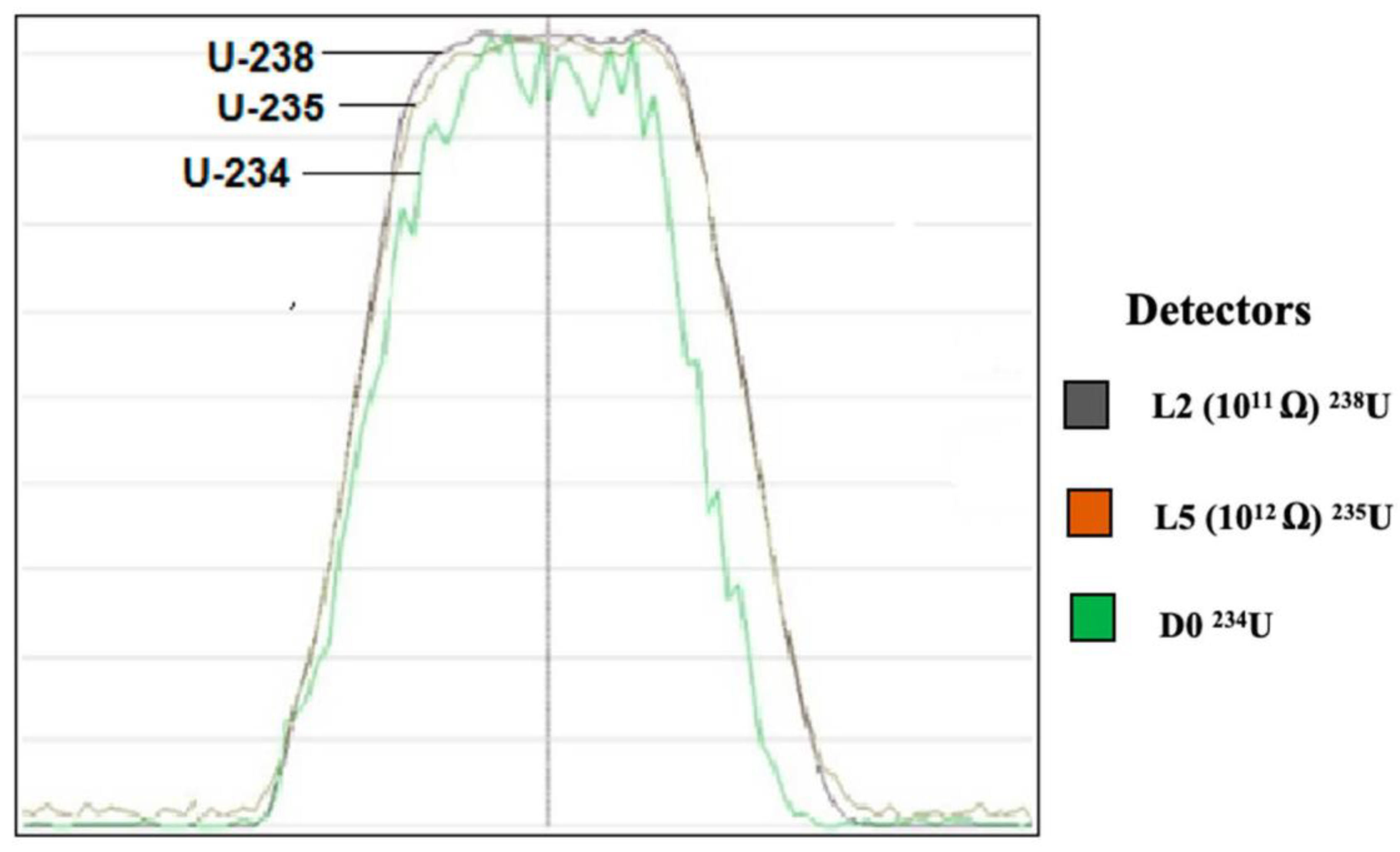
Uranium (U) is a naturally occurring radioactive element that has no stable isotopes but two primordial isotopes (uranium-238 and uranium-235) that have long half-life and are found in appreciable quantity in the Earth's crust, along with the decay product uranium-234. The average atomic mass of natural uranium is 238.02891(3) u. Uranium Ore samples are useful as check sources for testing Geiger Counters. No chemical or spectral analysis is performed on the radioactive ore sample. So the source of the ore's radioactivity is not determined and may be any of, or any number of radioactive elements such as uranium, thorium and potassium and any of their decay products such. Its pure form, uranium is a silver-colored heavy metal that is nearly twice as dense as lead. In nature, uranium atoms exist as several isotopes, which are identified by the total number of protons and neutrons in the nucleus: uranium-238, uranium-235, and uranium-234. (Isotopes of an element have.
- Uranium Isotopes Abundance
- Uranium Isotopes
- Uranium Isotopes Natural Abundance
- Uranium Isotopes Chart
- Uranium Isotope Masses
- Separation Of Uranium Isotopes
- Uranium Isotopes Atomic Mass
On this page:
Rule Summary
The Environmental Protection Agency (EPA) regulates radionuclides in drinking water to protect public health. Radionuclides in water at amounts greater than the drinking water standards may cause health problems.
On December 7, 2000, EPA published the Radionuclides Final Rule. The new rule revised the radionuclides regulation, which had been in effect since 1977. The revisions set new monitoring requirements for community water systems (CWS). This ensured customers receive water meeting maximum contaminant levels (MCL) for radionuclides in drinking water.
For more information on radionuclides in drinking water see the Basic Information page.
Radionuclides Rule Quick Reference Guide
This document provides a simple and straightforward description of the Rule. The guide includes critical deadlines and requirements for PWSs and states, and information on monitoring requirements.
- Radionuclides Rule: A Quick Reference Guide (PDF)(2 pp, 112 K, About PDF) EPA 816-F-01-003, June 2001
Rule History
In 2000, EPA revised the radionuclides regulation, which had been in effect since 1977. The revisions set new monitoring provisions for community water systems (CWS). This ensured that all customers of CWSs receive water meeting the maximum contaminant levels (MCL) for radionuclides in drinking water.
EPA also issued a standard for uranium, as required by the Safe Drinking Water Act (SDWA) Amendments of 1986. The current standards are:
- Combined radium 226/228 of 5 pCi/L;
- A gross alpha standard for all alphas of 15 pCi/L (not including radon and uranium);
- A combined standard of 4 mrem/year for beta emitters.
The new MCL for uranium is 30 µg/L.
The Office of Ground Water and Drinking Water's (OGWDW’s) Technical Support Center (TSC) develops analytical methods. These methods help determine the concentrations of chemical and microbial contaminants in drinking water.
EPA issued a final rule with three additional analytical methods. These include inductively coupled plasma mass spectrometry (ICP-MS) technology for compliance determinations of uranium.
In 2004, EPA published minor corrections to the Radionuclides Rule. The correction identified a detection limit for uranium and clarified Rule language.
EPA published a Notice of Data Availability (NODA). The NODA included new information that had become available since the 1991 proposed revisions to the 1976 Radionuclides Rule. The Agency also developed a technical support document to accompany the NODA.
This document provides background information and further describes the analyses discussed in the NODA. The document also contains a Preliminary Health Risk Reduction and Cost Analysis, which presents the analyses of:
- Projected impacts,
- Costs,
- Risk reductions, and
- Benefits for the uranium National Primary Drinking Water Rule (NPDWR) and the new monitoring requirement for radium-228.
In 1997, EPA held a public meeting to discuss regulatory issues associated with the 1991 Radionuclides Rule proposal.
In 1991, EPA proposed revisions to the July 9, 1976 Radionuclides Rule. The final Rule published in 2000 finalized this 1991 proposal.

Compliance
EPA has developed materials to assist drinking water system owners and operators, as well as States and Primacy Agencies. The links on this page will redirect to guidance and tools for the Radionuclide Rule.
Additional Tools and Resources
Radiation Protection Programs
Uranium Isotopes Abundance
EPA's Agency-wide Radiation Protection Program comprises seven groups of projects and issue-specific programs. You can find descriptions of the programs, as well as program contacts and news, on their webpage. The webpage also includes information on:
- laws and regulations,
- publications
- and technical materials.
Uranium Isotopes
A radioactive and strategic element
The uranium atom is the heaviest atom present in the natural environment. Its radioactivity is very low. Its very long life of several billion years has allowed uranium to be still present. It is a rare chemical element found in the Earth's crust with an average of 3 grams per tonne.
The uranium image has suffered from its association with the first atomic bombs. Its reputation as a malevolent radioisotope, however, is undeserved: in fact, the decay rate of uranium is among the slowest known to man. The activity of a sample of uranium could be compared to the water flow escaping from a pond through a pinprick.
Martin Heinrich Klaproth (1743-1817) was a German chemist best known for his discovery of a variety of chemical elements, including the 1789 discovery of uranium, zirconium and chromium. He is sometime credited as being the ‘father of analytical chemistry’.
DR
These reassuring features not prevent this unfortunate element to be regularly presented by TV channels as a dangerous radioactive substance ? Or is our complacence born of ignorance? Contrary to the widespread fears, uranium presents low risks owing to its very low radioactivity. Its radioactive toxicity, according to experts from the CEA, is a hundred times weaker than its chemical toxicity, which itself is no different from the chemical danger posed by common heavy elements such as lead.
Athabasca plateau in Saskatchewan province, Canada. Western Canada is particularly rich in uranium, with anywhere between 28 to 210 kilograms of uranium per tonne as opposed to the usual 3 grams per tonne found elsewhere. This high abundance, taken in conjunction with the difficult geological conditions and the harsh climate have made the extraction process an almost entirely automated one.
HARRY GRUYAERT /MAGNUM /AREVA
Though both isotopes were at the time of Earth formation equally abundant, natural uranium today consists today of 99.3% uranium 238 and only 0.70% uranium 235. The nuclei of uranium 235 and 238 are, along with those of thorium 232, the heaviest present in nature. They were all formed billions of years ago by the explosion of heavy stars (supernovae).
The radioactivity of uranium is low, and so no particularly high standards of radioprotection are needed: as can be seen with the above workmen. The concentrated uranium they are handling, also known as ‘Yellow Cake’, takes the form of a bright yellow powder containing about 750kg of uranium per tonne. This high concentration makes it much easier to transport the uranium from the mine to the factory. The photograph above shows the ‘yellow cake’ on a filter at a treatment plant in Jouac (Haute-Vienne) at Limousin, France.
PHILIPPE LESAGE/COGEMA
As a particularly heavy element, uranium isotopes are primarily alpha emitters, though these radiations are sometimes accompanied by gamma rays.
Natural uranium is poor in the fissile isotope, containing as it does only 0.70% of uranium 235. It must be enriched before it can be used as a fuel in any commercial reactor. These reactors are powered by uranium which is enriched to have anywhere between 3 and 4% of uranium 235. In order to have an atomic bomb, the uranium has to be enriched to above 90%. The boundary between the uranium meant for civilian uses (LEU or low-enriched uranium) and that uranium meant for military use (HEU or highly-enriched uranium) is generally fixed at 20%.
IN2P3
Uranium Isotopes Natural Abundance
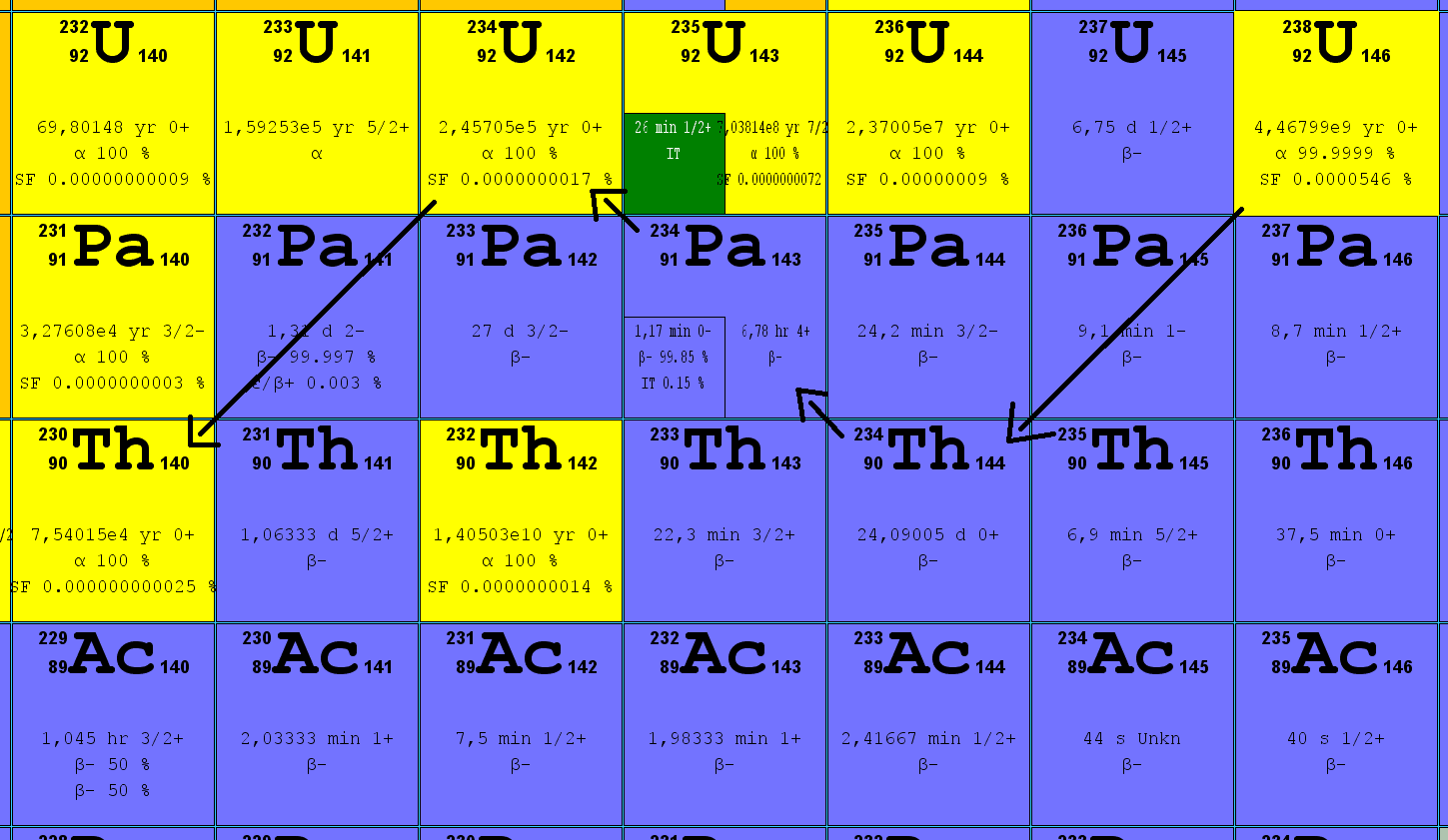
Uranium 235 is the only natural nucleus that can easily undergo fission. Highly sought-after, it can be used as a fuel in nuclear reactors and as an explosive in atomic bombs.The more abundant uranium 238 is sometimes called fertile. Fission occurs comparatively rarely, and even under bombardment with energetic neutrons the probability of fission remains very low. What happens more frequently is that a neutron capture causes the nucleus to become unstable. After a few days, uranium 239 has transformed into plutonium 239, a radioisotope with a half-life of 24,000 years. Plutonium 239 is highly fissile and can also be used as a nuclear fuel or an atomic explosive.

Uranium Isotopes Chart
Uranium reserves which exist under the oceans are about a thousand times more abundant than the reserves found in high quality minerals on land. Underwater uranium, however, would be extremely costly to exploit.TO KNOW MORE ABOUT URANIUM AND ITS USES :
- 1) :Isotopes de l'uranium
- 2) : Uranium fuels
Uranium Isotope Masses
- 3) : Propriétés de l'uranium
Access to page in french
Separation Of Uranium Isotopes
Uranium Isotopes Atomic Mass
Nuclear Fission
Fissile nuclei
Isotopic Separation
