Calcium is an alkaline earth metal and has been known since prehistoric times. It was discovered in 1787 by Antoine Lavoisier and was isolated in pure form in 1808. Calcium is important nutrient for the human body.
Plaque — made of fat, calcium and other substances — can build up and narrow or close the arteries. To detect this build-up, your physician may order cardiac calcium scoring — a test that is also known as coronary artery calcium (CAC) scoring, a heart scan or calcium score. The procedure is performed at the University Imaging Center at UMMC. In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom (there are 20 electrons). When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. In writing the electron configuration for Calcium the first two electrons will go in the 1s.

Discovery and History

The history of calcium dates to 7000 BC, when calcium in the form of lime was used as plasters for making statues and as building material. The name calcium is derived from the word “calx” that is Latin for lime. Another form of calcium, calcium sulphate that is termed as gypsum have been found to be used in the construction of Great Egyptian Pyramid of Giza. Later in 1787, lime was considered as an oxide of a novel chemical element by Antoine Lavoisier. Calcium was first isolated by Sir Humphry Davy in 1808 in England. He electrolyzed a mixture of lime and mercuric oxide [1]. A few other scientists, Magnus Pontin and Jöns Jacob Berzelius also produce a calcium amalgam after performing electrolysis on a mixture of lime and mercury oxide.
Calcium
| Periodic Table Classification | Group 2 Period 4 |
|---|---|
| State at 20C | Solid |
| Color | Silvery-gray |
| Electron Configuration | [Ar] 4s2 |
| Electron Number | 20 |
| Proton Number | 20 |
| Electron Shell | 2, 8, 8, 2 |
| Density | 1.55 g.cm-3 at 20°C |
| Atomic number | 20 |
| Atomic Mass | 40.08 g.mol -1 |
| Electronegativity according to Pauling | 1.00 |
Occurrence
Calcium is very reactive and does not occur in free form. It occur in earth’s crust in the forms of carbonate, sulfate, fluoride, silicate and borate. The calcium carbonate occurs in marble, chalk, limestone and calcite. Calcium sulfate (CaSO4) occurs in anhydrite and gypsum, calcium fluoride in fluorspar or fluorite (CaF2) and calcium phosphate occurs in apatite. Calcium also occurs in numerous silicates and alumino silicates. Almost all natural waters, including seawater, contain either or both calcium carbonate and calcium sulfate. Many organisms concentrate calcium compounds in their shells or skeletons. For example calcium carbonate is formed in the shells of oysters and in the skeletons of coral. [2]
Physical characteristics
Its physical and chemical properties are most similar to strontium and barium. It is the fifth most abundant element in Earth’s crust and the third most abundant metal, after iron and aluminum. The atomic number of calcium is 20, and atomic weight is 40.078. The density of calcium is 1.55 grams per cubic centimeter. Its melting point is 842 °C and boiling point is 1494 °C. Calcium is harder than lead but can be cut with a knife with effort. Calcium is a poorer conductor of electricity than copper or aluminum (by volume), but it is a better conductor by mass due to its very low density. [3] It reacts with atmospheric oxygen, which makes its unfavorable to be used in the most applications, but its usage is space is being considered such in space. [4]
Chemical characteristics
The chemistry of calcium is similar to heavy alkaline earth metal. The metal reacts slowly with oxygen, water vapor, and nitrogen of the air to form a yellow coating of the oxide, hydroxide, and nitride. It burns in air or pure oxygen to form the oxide and reacts rapidly with warm water to produce hydrogen gas and calcium hydroxide. On heating, calcium reacts with hydrogen, halogens, boron, sulfur, carbon, and phosphorus. Although it compares with sodium as a reducing agent, calcium is more expensive and less reactive than the latter. In many deoxidizing, reducing, and degasifying applications, however, calcium is preferred because of its lower volatility and is used to prepare chromium, thorium, uranium, zirconium, and other metals from their oxides. [5] Calcium metal dissolves directly in liquid ammonia to give a dark blue solution. [6] Due to the large size of the Ca2+ ion, high coordination numbers are common.
Uses and Significance
- Calcium carbonate is taken as an antacid is effective for treating indigestion.
- Giving calcium gluconate intravenously (by IV) can reverse hyperkalemia, a condition in which there is too much potassium in the blood.
- Taking calcium by mouth is effective for treating and preventing hypocalcemia. It is also given intravenously (by IV) for treating very low levels of calcium in the body.
- Taking calcium carbonate or calcium acetate by mouth is effective for controlling high phosphate levels in the blood, that is present in people with kidney failure.
- Taking calcium by mouth is effective for preventing bone loss and treating osteoporosis.
- Calcium is a co-factor for many enzymes which makes it’s a vital component of the biological system.
- Calcium affects the smooth muscle that surrounds blood vessels and cause it to relax. There are various ionic channels in the membrane of living cells that are controlled by level of calcium in the body.
- It is important to note that calcium is not easily absorbed without the presence of vitamin D.
Isotopes
There are six natural isotopes of calcium, including; Ca-40 is most abundant (97 percent of natural abundance), Ca-44 (2 percent of natural abundance); Ca-42 (0.6 percent of natural abundance); Ca-48 is most stable (0.2 percent of natural abundance); Ca-43 (0.1 percent of natural abundance); Ca-46 (0.004 percent of natural abundance). It is the first element to have low density and have six natural isotopes.
References
1. https://education.jlab.org/itselemental/ele020.html
2. http://nautilus.fis.uc.pt/st2.5/scenes-e/elem/e02020.html
3. Ropp, Richard C. (31 December 2012). Encyclopedia of the Alkaline Earth Compounds. pp. 12–5. ISBN 978-0-444-59553-9.
4. Hluchan and Pomerantz, p. 484
5. https://www.britannica.com/science/calcium
6. Greenwood and Earnshaw, pp. 112–3
Other Periodic Table Elements
- Erbium
Erbium was discovered in 1843. Its pink colored Er3+ ions have fluorescent properties useful in…
- Holmium
Holmium was discovered in 1878. It has highest magnetic moment that is why it is…
- Francium
Francium was discovered in 1939. It is very unstable alkali metal and considered the second…
Molar mass of Ca = 40.078 g/mol
Convert grams Calcium to moles or moles Calcium to grams
| Symbol | # of Atoms | Calcium | Ca | 40.078 | 1 | 100.000% |
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.

Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.

A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Calcium Mass Number Of Most Common Isotope
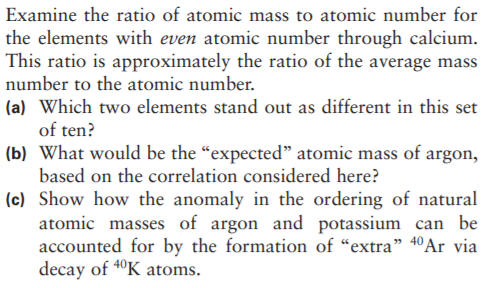
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Calcium Mass Number Amu
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
